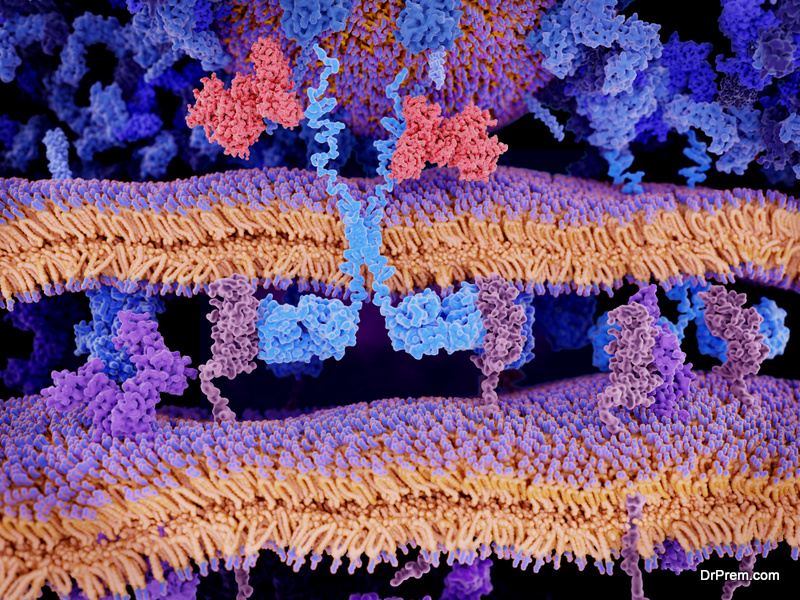
Two of the most relevant measurements to understand when exploring protein interactions are binding affinity and binding kinetics. The study of both metrics enables scientists to explain biological processes and to draw complex metabolic pathways. In fact, without these two measurements, efficient drug development would not take place, making it impossible for the pharmaceutical industry to treat a vast number of diseases.
Measurements that Provide Us with Vital Information

Binding affinity represents the strength of the binding interactions between a biomolecule and its ligand. Strong interactions can be characterized as picomolar affinities; while on the other hand, weak interactions can be in the millimolar range.
Binding affinity is represented by the equilibrium dissociation constant (Kd). The smaller the Kdvalue, the higher the binding affinity of the biomolecule to the ligand1. The attraction is determined by non-covalent molecular interactions such as hydrogen bonding, electrostatic interactions, hydrophobic and Van der Waals forces. Other molecules may also affect the binding affinity between two biomolecules operating as modulators of the interaction.
MicroscaleThermophoresis measures the strength of these interactions by utilizing a fluorescently labeled or intrinsically fluorescent sample and a binding ligand, which is measured while a temperature gradient is applied. MicroscaleThermophoresis is perhaps the most useful method that helps to obtain precise and quantitative values of the affinity between two biomolecules.
Binding kinetics on the other hand, describes how fast a ligand binds to its target and how fast it dissociates from it, also known as measuring the on-rate and off-rate. Measuring the on-rate and the off-rate has always been complex, but new technology has substantially contributed to the acquisition of more accurate results. The development of biophysical techniques such as surface plasmon resonance has allowed for the measurement of on- or off-rates in a faster way. This optical method requires an efficient binding of the mobile molecule (analyte) to a molecule immobilized on a thin metal film (ligand).
Surface plasmon resonance has also become a commonly used technique due to the many advantages that it presents. One of the benefits, for example, is that the ligand can be captured directly from complex samples involving less manipulation.
The Impact of Binding Affinity and Binding Kinetics in Research

The utilization of the aforementioned technologies to measure binding affinity and binding kinetics has revolutionized the pharmaceutical industry, primarily drug discovery.
Measuring binding affinity and kinetics has allowed the pharmaceutical industry to engineer drugs to replace unstable abnormal proteins or molecules that operate as inhibitors or that trigger signaling in a defective network due to loss of activity from affected proteins. It has also contributed to the understanding of the regulatory pathways that occur in the body. These are just some of the ways that measuring binding affinity and binding kinetics are necessary for the advancement of pharmaceutical research.
Article Submitted By Community Writer